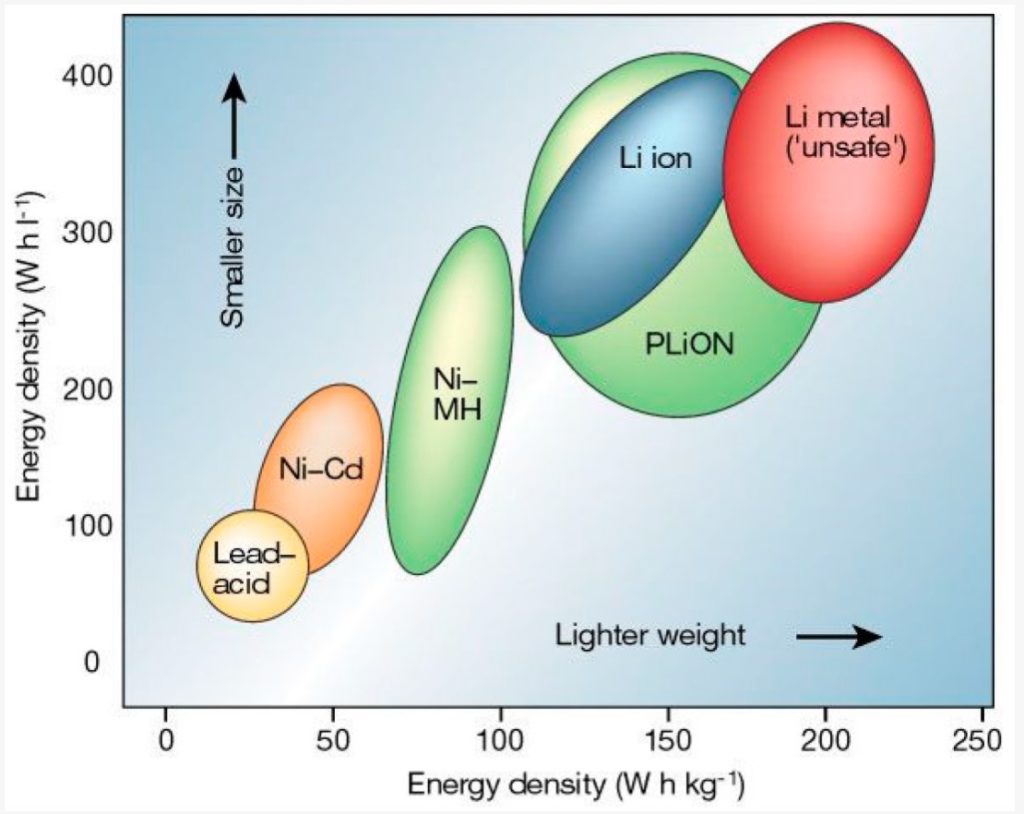
The difference between lithium ion and lithium polymer batteries
A lithium-ion polymer (LiPo) battery (also known as Li-poly, lithium-poly, PLiON, and other names) is a rechargeable Li-ion battery with a polymer electrolyte in the liquid electrolyte used in conventional Li-ion batteries. There are a variety of LiPo chemistries available. All use a high conductivity gel polymer as the electrolyte. LiPos provide higher specific energies than other lithium batteries, often used in systems where weight is an important factor, such as mobile devices, drones, and some electric vehicles. This FAQ begins with a high-level comparison of Li-ion and LiPo batteries, followed by a detailed look at the six basic lithium battery chemistries most suitable for use in LiPo batteries. It closes with a look into the future and the possible development of aluminum-air polymer batteries and solid-state batteries.
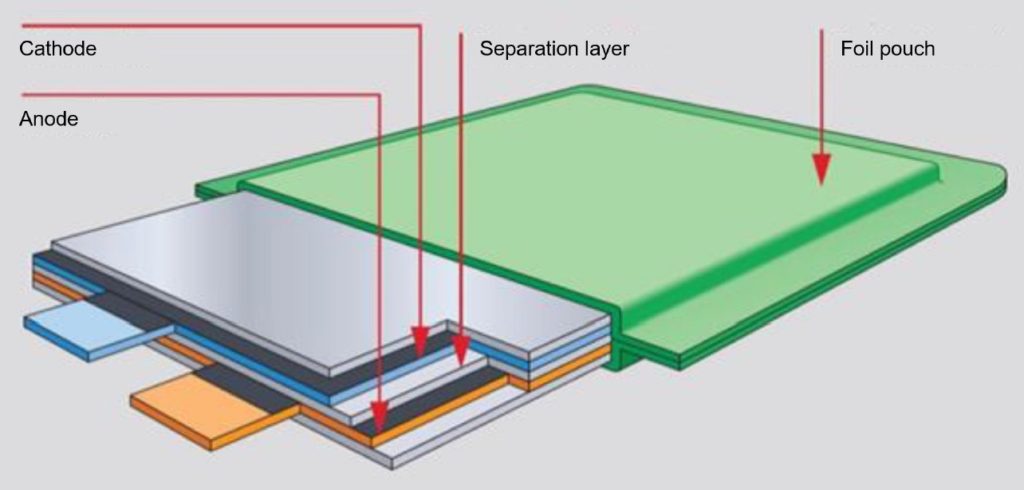
All lithium batteries include a barrier to separate the anode and cathode while also enabling the movement of ions between the electrodes. In a LiPo, the polymer separator also contains the electrolyte. In addition, polymer separators can provide an additional function acting as “shutdown separators” that can shut down the battery if it becomes too hot during charging or discharging. Shutdown separators are multilayer structures with at least one polyethylene layer which can stop current flow when the temperature rises too high and at least one polypropylene layer which acts as a form of mechanical support for the separator.
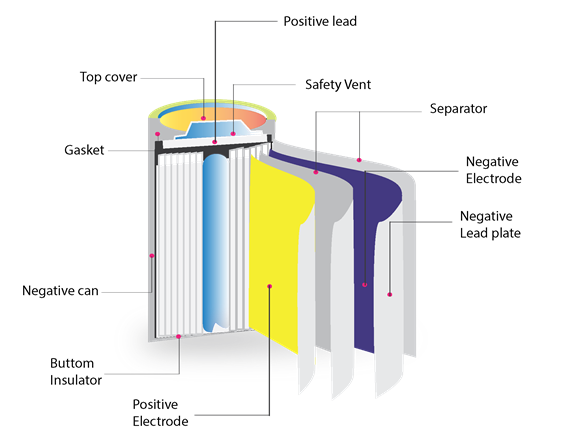
The intercalation and decalation of lithium ions from a positive electrode and a negative electrode. Except for the polymer separator, LiPos operate on the same principle as Li-ions. However, they are packaged in quite different ways.
Li-ions are usually delivered in a stainless steel or aluminum case. The case is most often cylindrical but can be button-shaped or rectangular (prismatic). The case is relatively costly to produce and tends to restrict the sizes and shapes that are available. But it is also robust, helping to protect the battery from damage. The case is sealed using a laser welding process.
LiPos are packaged in an aluminum foil “pouch” and are called soft or pouch cells. The pouch is mostly prismatic and easier to fabricate, and lower in cost than the stainless steel or aluminum cases of Li-ions. This type of construction also enables the production of batteries with a variety of custom configurations. The other components in LiPos include wafer-thin layers (< 100 μm) that can be mass-produced at a relatively low cost. Substituting the foil pouch for the metal can result in high energy density and lightweight batteries. Both large formats and heights of less than 1 mm can be achieved, but the cells require careful mechanical handling.
The use of LiPos is subject to many of the same challenges that users of Li-ion must contend with, including overcharging, over-discharging, over-temperature operation, and internal shorts. In addition, crushing or nail penetration of the LiPo pouches can result in catastrophic failures ranging from pouch ruptures to electrolyte leaks and fires.
Like Li-ions, LiPos can expand at high levels of overcharge due to the vaporization of the electrolyte. Vaporization of the electrolyte can cause delamination, causing bad contacts between the internal layers of the cell, reducing reliability and cycle life. This expansion can be particularly noticeable for LiPos, which can literally inflate. It can also cause structural damage to the host system.
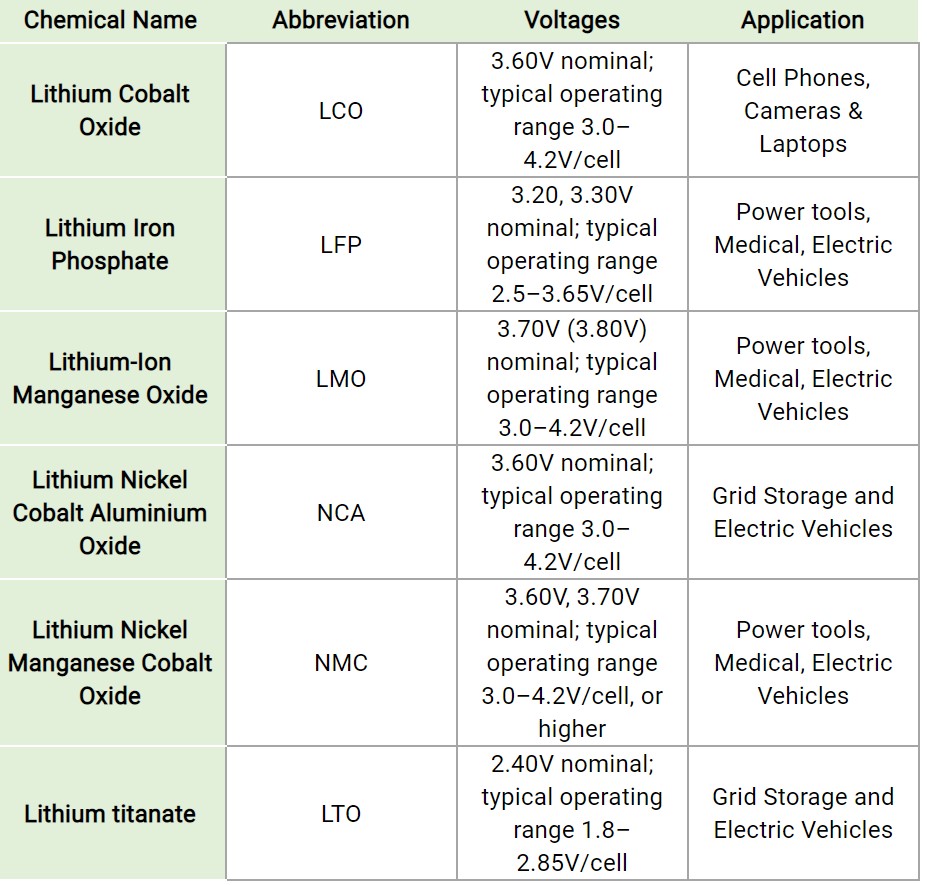
The table below compares the voltages and typical applications of the six basic lithium battery chemistries. Other characteristics of these batteries include:
- LCO – 200Wh/kg, deliver a high power, but with the tradeoff of relatively short lives, low power ratings, and low thermal stability.
- LFP – 120Wh/kg, have long cycle lives and stability at high operating temperatures.
- LMO – 140Wh/kg, cathodes are based on manganese-oxide components that are abundant, inexpensive, non-toxic, and provide good thermal stability.
- NCA – 250Wh/kg, offers high specific energy and long cycle lives.
- NMC – 200Wh/kg, varying the proportions of the chemical constituents allows the development of batteries optimized as power or energy cells. Because of its flexibility, it is one of the most successful lithium battery chemical systems.
- LTO – 80Wh/kg, lowest specific energy, but can be fast charged, discharged at up to 10-times its rated capacity, and is safe.
Note that the NMC, LCO, and NCA batteries contain Cobalt that helps to provide higher power capabilities. They can provide large amounts of power in a small package but can be more susceptible to thermal events that can cause safety issues.
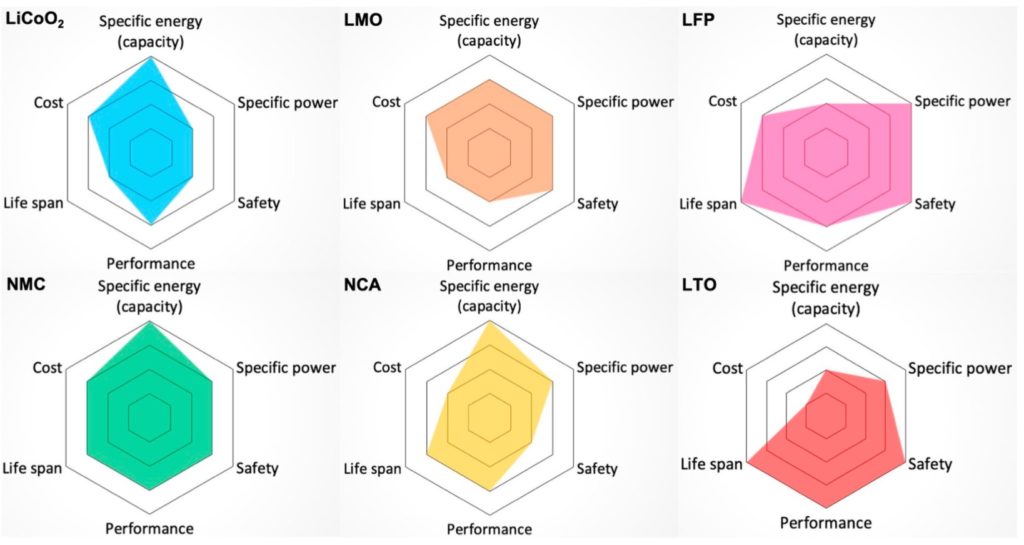
The next figure includes spider diagrams comparing the basic types of Li batteries based on their suitability for use in electric vehicles (EVs). In these spider diagrams, batteries that are better suited for EVs have a larger colored area. The factors considered are specific energy, specific power, safety, performance, life span, and cost. Specific energy in Wh/kg relates to the EV range. Specific power in W/kg relates to EV acceleration. In the case of EVs in particular, safety is a critical consideration. The Performance parameter reflects the battery’s ability to be used in extreme temperature conditions, also an important consideration in automotive applications. Life Span is a combination of cycle life and longevity. Cost attempts to capture all related costs, including ancillary systems for thermal management, safety, battery management, and monitoring, and the need for an extended warranty period in EVs.
LiPo chemistries
A polymer electrolyte results in several performance enhancements, including high energy density and lightweight batteries. Depending on the structure of the polymer layers, it can also enhance battery safety. Compared with conventional Li-ion batteries, LiPo batteries can be fabricated with a wider range of specific energy densities (Wh/kg) and specific power densities (W/kg), making LiPo batteries more flexible across a wider range of potential applications. As a result, LiPo technology is used across all the main lithium battery chemistries:
- Lithium cobalt oxide battery (LCO)
- Lithium-ion ternary battery (NCA, NMC)
- Lithium-ion manganese oxide battery (LMO)
- Lithium iron phosphate battery (LFP)
Aluminum-air and solid polymer batteries
Aluminum-air polymer batteries are under active development. These high energy density designs have a polymer separator directly contacted with the lithium anode to separate it from the cathode. As in other polymer batteries, the separator prevents the battery from short-circuiting and absorbs liquid electrolyte to support ion transport and complete the electrical circuit.
Unfortunately, the lithium anode can form dendrites during battery cycling. These dendrites can penetrate the polymer separator and shorten the battery. Modified separators are under development that includes graphene oxide layers. The graphene oxide protects the anode from contaminates and prevents chemical fluctuations on the surface of the lithium anode. The graphene oxide works together with the polymer layer to stop direct contact between the electrolyte and the lithium anode without significantly reducing ion conductivity. This combined structure slows electrolyte corrosion on the anode. It is hoped that in the future, the use of two types of layers to stabilize the lithium anode will result in very high energy density batteries with reasonable cycle lives.
Cells with truly solid polymer electrolytes (SPE) in place of today’s gelled membranes are also under development. Today’s LiPo cells are considered a ‘hybrid’ system between a conventional Li-ion and a completely solid-state Li-ion battery. Gelled membranes are hybrid systems where the liquid phases are contained within the polymer matrix. While they may feel dry to the touch, they can contain up to 50% liquid solvents. Today’s systems are also called hybrid polymer electrolyte (HPE) systems that combine the polymer material, the liquid solvent, and salt. SPEs are under development that are completely solvent-free systems in a polymer medium.
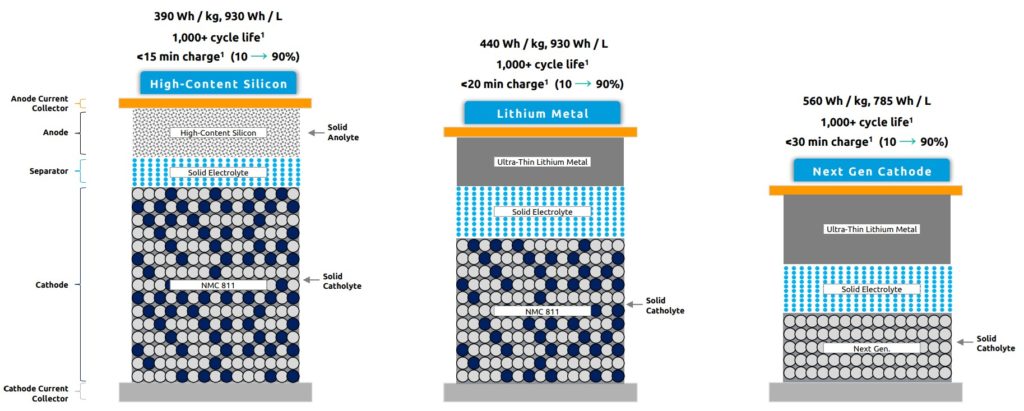
The new solid-state structure can also use low-cost and high specific energy conversion type cathodes that are not compatible with liquid-based battery chemistries such as lithium-ion. One example is a proprietary sulfide solid electrolyte that supports high-content silicon and lithium metal in the anode paired with industry-standard and commercially mature cathodes, including lithium nickel manganese cobalt oxides (NMC). The new cathodes can be combined with lithium metal to remove cobalt and nickel and could decrease cathode active material costs by 90%.
Solid-state cells have been produced, delivering 2Ah using industry-standard lithium-ion equipment and processes. Commercial production of a 20Ah high-content silicon anode cell is expected by the end of 2021, with 100Ah expected to follow in 2022.
Summary
LiPos offers several performance enhancements compared with Li-ions, including higher energy density and lighter-weight batteries. In addition, LiPos can be produced in a wider variety of shapes and sizes. However, today’s LiPos use gelled membranes, not fully solid polymer electrolytes (SPEs). SPEs are under development and could extend the performance advantages of LiPos in certain applications. Aluminum-air polymer batteries offer the potential for very high energy densities (resulting in longer ranges for EVs) and good cycle lives. Completely solid-state large format lithium batteries are on the horizon for later in 2021.